Research
Rehabilitation for people with diseases and disabilities, health promotion exercises for healthy people, and training for athletes are all “physical exercise,” regardless of the degree or type, and are performed mainly by exerting force through contraction of skeletal muscles. The main part of the exercise is the exertion of force through contraction of skeletal muscles. The basic principles of “the more you use it, the stronger and larger it becomes,” “if you don’t use it, it will deteriorate,” and “if you use it too much, it will malfunction” (the three classical Rouxian principles) apply to the musculoskeletal system, with skeletal muscles in particular, and this property of being able to change is referred to as “plasticity. Our laboratory is conducting research using physiological, biochemical, and molecular biological techniques on humans, laboratory animals, and cultured cells to elucidate the regulatory mechanisms of plastic changes in the locomotor system associated with various changes such as exercise, aging, inactivity, nutritional status, and disease onset.

(green: laminin, red: slow myosin heavy chain)
Intracellular regulatory mechanisms that modulate quantitative and qualitative changes in skeletal muscle.
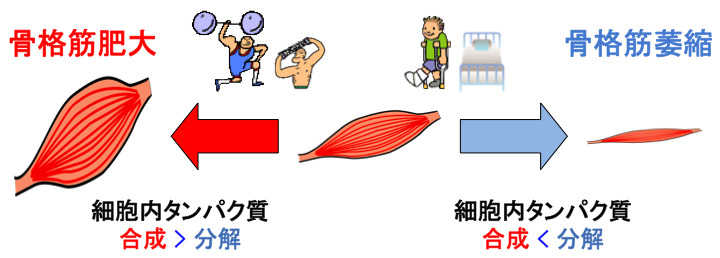
Skeletal muscle is a population of myofibers, which are terminally differentiated multinucleated cells, and the total number of myofibers does not change significantly in mature skeletal muscle. Quantitative changes in the tissue as a whole, such as an increase (muscle hypertrophy) or decrease (muscle atrophy) in skeletal muscle mass, represent a change in the size of individual myofibers. This change in myofiber size is defined by the balance of protein synthesis and degradation within the cell. In other words, an increase (or decrease) in skeletal muscle mass can be defined as “a state in which the amount of protein synthesis in skeletal muscle cells exceeds (falls below) the amount of protein degradation. In our laboratory, we are conducting research using various genetically engineered animals and cell culture systems to elucidate the regulatory mechanisms of muscle protein metabolic changes associated with exercise training and inactivity.
Why Don’t Hibernating Animals Become Bedridden?
~Mechanisms of acquisition of skeletal muscle atrophy tolerance in hibernating animals
In the case of human skeletal muscle, muscle protein and exerted muscle strength decrease at a rate of about 0.5-1.0% per day after inactivity such as bed rest, accelerating the loss of muscle mass. However, in hibernating animals, despite experiencing prolonged inactivity and malnutrition (fasting) for approximately six months, muscle weight and exerted muscle strength either do not change at all before and after hibernation (in squirrels, Andres-Mateos et al., EMBO Mol Med 2013) or decrease to some extent but very minimally compared to humans In the case of bears (Miyazaki et al., PLOS ONE 2019), there is an unexplored physiological function that can be called skeletal muscle atrophy tolerance. On the other hand, it has also been shown that even hibernating animals lose significant muscle mass when activity is restricted during the non-hibernation period (Lin et al., J Exp Biol 2012). In other words, the muscle mass maintenance mechanism in hibernating animals can be considered an adaptive system resulting from some physiological response induced by hibernation. We are focusing on the characteristic of “undiminished muscle mass” possessed by hibernating animals, and by elucidating its acquisition mechanism, we aim to ultimately develop methods to prevent the onset of disuse syndrome and sarcopenia in humans, and propose effective rehabilitation methods.





